
*Notice- 21 Nov 2024
IACUC/2024/09
Dear PIs,
Please be informed that your IACUC submissions for review at the 20 Jan 2025 IACUC meeting should reach us/be submitted by Mon, 9 Dec 2024.
The Jan 2025 submission deadline is advanced to 9 Dec 2024 because of the year-end holidays. This is a one-off adjustment which will not affect the submission cut-off dates for other months.
Applications received between 10 Dec 2024 and 20 Jan 2025 (date inclusive) will be reviewed at the 17 Feb 2025 IACUC meeting.
Thank you.
IACUC office
*Notice- 25 Oct 2024
IACUC/2024/08
Dear PIs,
- Due to some technical issues during the backend uploading of user role, all staff and graduate students have been assigned the PI role in the iRIMS-IACUC (Animal Oversight module). We are looking for a solution to the above. In the meantime, we would appreciate if you could inform your staff to contact the IACUC office immediately if they are not able to locate your name under the “PI group” assignment (see screenshot below). Please DO NOT start any application under the wrong PI group. There will be problem changing PI once the application is drafted.
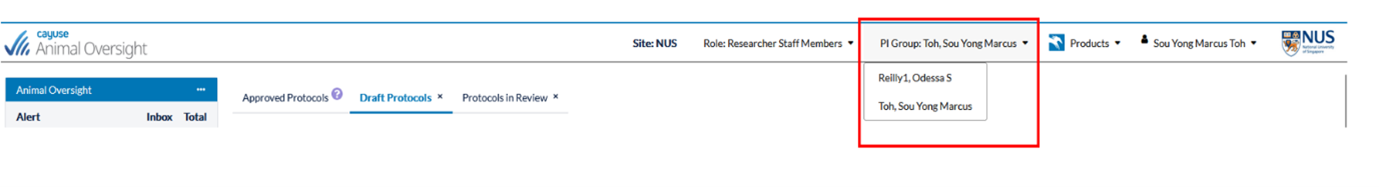
- We would also like to bring to your attention that under the iRIMS-IACUC (Animal Oversight) module,
a. All staff and graduate students under the same PI’s group will have access to all the PI’s applications regardless of whether he or she is listed as a team member in a specific application.
b. Undergraduate students will not have access to iRIMS-IACUC. Please write in to the IACUC office to create an account for them and place them under a PI group before adding them to an IACUC protocol.
c. Generally, only the PI can submit an application. If needed, PIs can write to the IACUC office to authorise a team member to submit the application and subsequent amendments on their behalf. Please find below a summary of the various roles in the system and make your nomination in each application accordingly.
| Role | Details
|
| PI | The person who proposes and/or has IACUC approval to conduct a protocol involving the use of animals, and has overall responsibility of a protocol. If needed, please refer to attached IACUC policy P202 for details on who can be a PI.
|
| Team member authorize by PI to submit an application and subsequent amendments on his behalf | A listed team member in a specific application whom the PI has given the authority to handle online administrative matters relating to the protocol. This includes the submission of online forms for IACUC office processing. Please note that this team member must obtain RCULA/RCUF training and enrol in NUS OH programme even if he/she is not handling live animals. PI will need to write to the IACUC office for such authorization. |
| Primary contact | A listed team member who will receive email notification when action needs to be taken for an iRIMS-IACUC application.
Please make sure both the "Primary Contact" and "Copy Primary Contact on all emails" boxes are checked in the online form for this function to work properly.
|
| Emergency contact | - A person the PI has nominated and authorized to provide oversight and make decisions on the day-to-day running of the protocol. The PI still has ultimate responsibility for the protocol and/or
- A person the PI has nominated and authorized to make decisions on non-compliance and animal welfare issues.
|
| Team member | A person listed and approved by IACUC to conduct specific procedures and duties in an approved protocol. “Standard” team members will be able to make changes to the online iRIMS-IACUC forms but do not have the authority to submit the forms to IACUC office for processing. |
d. New staff/student must be added to the PI’s group before the new staff/student can access iRIMS-IACUC. Please contact the IACUC office to add the new staff/students to your PI’s group.Thank you
IACUC Office
*Notice- 13 Sept 2024
IACUC/2024/07
Dear Principal Investigators,
In conjunction with the launch of the iRIMS-IACUC (Animal Oversight) module on 17 September 2024, please note the following points:
- New IACUC applications submitted via the iORC system after 16 September 2024, 18:00 hrs will not be accepted and Principal Investigators must create and submit an application in iRIMS-IACUC (Animal Oversight) module.
- Subsequent amendment, renewal and closure of any iRIMS-IACUC application will have to be made through the iRIMS-IACUC (Animal Oversight) module.
- For IACUC applications which have already been approved in the iORC, amendments to them must be made and submitted via the iORC until the application is closed or expired.
- Any protocol that has been reviewed by the IACUC more than a year ago but not approved will be removed in the iORC. You are required to submit a new application via iRIMS-IACUC (Animal Oversight) module for IACUC to review it. This is in accordance with the IACUC SOP in which applications that have not been approved within a year from the IACUC meeting date will have to be re-submitted for IACUC review.
You can refer to the Joint-Circular from IACUC and IBC for more information.
If you have any question, please feel free to either email IACUC General Enquiries and Self Report Submissions or contact any of the IACUC office staff here
We would be grateful if you could disseminate the information to your lab members
Thank You
IACUC Office
*Notice- 20 Aug 2024
IACUC/2024/06
Dear PIs
Townhall by IACUC on Thurs 29 Aug 2024 at 3PM, CeLS Auditorium
You are cordially invited to attend the Townhall session on 29 August 2024 (Thursday) at 3pm, CeLS Auditorium. The tentative agenda is below.
Agenda:
3.00 pm: Welcome & Introduction by Prof Sanjay Khanna (IACUC Chair)
3.10 pm: Presentation by IACUC Office - Timeline of protocol review, Avoiding non-compliances
3.40 pm: Presentation by Dr Leslie Retnam (Attending Veterinarian and Director, CM) -‘Partnering with CM for your animal research needs’
4:10 pm: Responses to submitted questions*
4:30 pm: Questions and Answers session
5:00 pm: Closing Remarks
5:15 pm: End of Townhall
You may submit questions anonymously for IACUC at https://admin.sli.do/event/wH55XPAq3LQVtYq5fucNaK/questions.
If you and/or your research staff intend to attend, please RSVP by providing names, depts or programmes to IACUC Office at dprbox33@nus.edu.sg by 21 August 2024 (Wednesday).
** Notice- 28 May 2024
IACUC/2024/05
Dear PIs
Please be informed that your iORC submissions for review at the IACUC meeting on 15 Jul 2024 should reach us/be submitted by Thurs 13 Jun 2024.
The July deadline for submissions is advanced to 13 Jun because of the public holiday on 17 Jun 2024. This is a one-off adjustment which will not affect the deadlines for other months.
Review of applications received beyond 13 Jun 2024 will be deferred to 19 Aug 2024.
Thank you.
IACUC Office
** Notice- 17 May 2024
IACUC/2024/04
- NACLAR Guidelines 2022 (pg 9, para II.4.3.4) states that “Breeding of animals must be managed to avoid, or minimise, the production of excess animals”. To facilitate the review and approval of animal breeding, IACUC has introduced a template for section 2.8 of Breeding protocols (see attached). It is also anticipated to help to reduce the number of questions raised on the alignment of strains and animal number between research and breeding protocols and amendments during IACUC review. Do feel free to provide feedback and suggestion for improvement on the template, if any. Please be informed that the attached template need to be completed and submitted with the following:
- all new breeding protocols including renewal; and
- any amendments to breeding protocols that involved addition of strains or changes in animal numbers.
- IACUC would also like to remind PIs and researchers that the animal numbers must be similarly aligned in both research and breeding protocols and amendments (see point 1 above), with a permissible variation of not more than 5% or a maximum of 50 whichever is the lower.
- In addition, NACLAR Guidelines 2022 (pg 66, para III.4.7.4) states that “For research purposes, i.e. when the compounds are used to accomplish the scientific aims of the study, pharmaceutical-grade substances must be used if available, and suitable”. Hence, PIs are advised to use pharmaceutical grade substances for in vitro studies if they planned to eventually moved into in vivo animal model.
Thank you for your kind attention and we wish you all the best in your work with animals !!
IACUC office (on behalf of IACUC)
** Notice- 15 Mar 2024
IACUC/2024/03
Latest version of self-report form is now available on IACUC website
The latest version of the self-report form used to inform the IACUC whenever there is any adverse event, and/or unanticipated problems inlcuding the unexpected deaths of animals, possible non-compliance or any other issues relating to welfare during the care and use of animals is now available on IACUC website
Thank you.
IACUC office